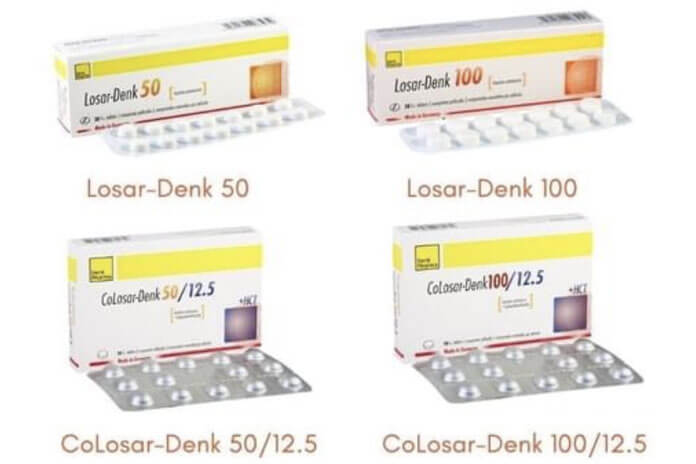
BELMOPAN, Wed. Oct. 6, 2021– The Ministry of Health and Wellness this week received a letter from the pharmaceutical company Denk Pharma GMBH & Co. KG, which advised the Ministry to conduct a voluntary recall of Losar-Denk (Losartan) and CoLosar-Denk (Losartan/Hydrochlorothiazide) medications from the Belizean market.
The medications are generally used to treat high blood pressure and heart failure, but European health authorities identified traces of a possible carcinogenic impurity “4-Chlor-Azidomethyltetrazole” within the active ingredient in the medication, Losartan — thus prompting the recommendation for a recall of the meds.
An AMES test (a mutagenicity test) that was conducted on the pills had yielded a positive result — indicating the presence of the impurity, which is a genotoxic substance. There currently is no clinical data on the impact of the impurity on humans, but based on investigations conducted by the Denk Pharma GmbH & Co. KG, the voluntary recall was deemed to be the best course of action.
European authorities have stated that the impurity was formed in the production of Losartan and is associated with the active ingredient of at least three manufacturers. The concentration of the impurity exceeded the Threshold of Toxicological Concern (TTC) limit. Based on TTC guidelines, it was recommended that products containing this genotoxic be recalled to limit carcinogenic risk.
Guided by this recommendation, the Health Ministry is encouraging the public to discontinue use of the listed medications and to discard those meds or return them to the nearest pharmacy. In Belize, the only importer registered to import from the Denk manufacturer is James Brodie & Co. Ltd. The company issued a release which stated, “Customers should return the following medications in their box to any of our pharmacies, where a voucher will be given in return.”
Denk Pharma has committed to covering the entirety of the costs relating to the recall, including the costs of destruction and the value of the goods. As a result, James Brodie and Co. Ltd. urges affected persons to provide information at the soonest convenience, but before the end of 2021 to allow for a credit note to be filed.
The Denk Pharma GmbH & Co. KG is yet to receive official reports of adverse events associated with the impurity. The German Federal Institute for Drugs and Medical Devices (BfArM), however, mentioned that preliminary assessments indicate a significantly lower health risk as opposed to nitrosamines. According to Denk Pharma GmbH & Co. KG, the available data suggests that the risks of untreated cardiovascular disease may outweigh the potential risks of this impurity.